Re: Document Hierarchy Structure
Hi Rose,
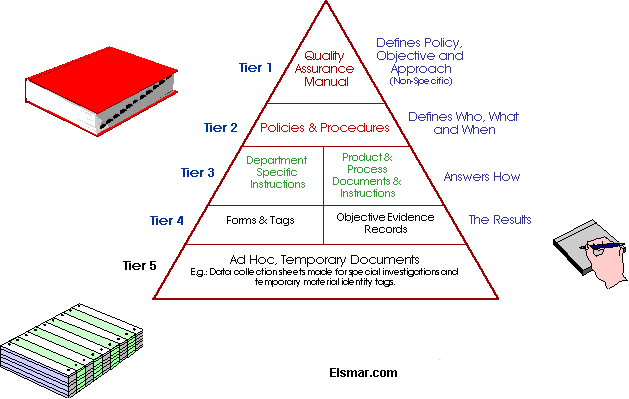
The documentation structure typically consists of 4 levels -
Level 1 - Quality Manual
Level 2 - Documented procedures required by the standard (eg. ISO
9001:2000 requires 6 mandatory procedures)
Level 3 - Documents needed by the organisation to ensure effectice
planning, operations and control of processes.
Level 4 - Records
I am attaching the "ISO 9000 Introduction and Support Package:
Guidance on the Documentation Requirements of ISO 9001:2000"
which you will find it very useful. (Special thanks to Sidney Vienna for contributing this in the Cove)
On the document numbering, there are a variety of document numbering styles. Typically, ensure that the owning dept and ISO clause is covered for easy tracebaility. For eg - ABC - 3702 - EN - 001, where ABC is the company initials, 3 stands for Level 3 and 702 represents the ISO Clause, EN stands for Engineering and 001 stands for the sequential numbering within that particular clause and for that department.
Likewise, the Level 2 and Level 4 numberings can be done.
Is Level 1 is Quality Manual or Quality Policy ?
Since
Guidance on the Documentation Requirements of ISO 9001:2000"
describes the following
 4 Guidance on Clause 4.2 of ISO 9001:2000
4 Guidance on Clause 4.2 of ISO 9001:2000
The following comments are intended to assist users of ISO 9001:2000 in understanding the intent of the general documentation requirements of the International Standard.
a) Documented statements of a quality policy and objectives:
- Requirements for the quality policy are defined in clause 5.3 of ISO 9001:2000. The documented quality policy has to be controlled according to the requirements of clause 4.2.3. Some organizations may be revising their quality policy for the first time, in order to meet ISO 9001:2000 requirements, and will need to pay particular attention to clause 4.2.3 (c), (d) and (g).
- Requirements for the quality objectives are defined in clause 5.4.1 of ISO 9001:2000. These documented quality objectives are also subject to the document control requirements of clause 4.2.3.
b) Quality Manual:
- Clause 4.2.2 of ISO 9001:2000 specifies the minimum content for a quality manual. The format and structure of the manual is a decision for each organization, and will depend on the organization’s size, culture and complexity. Some organizations may choose to use the quality manual for other purposes besides that of simply documenting the QMS
- A small organization may find it appropriate to include the description of its entire QMS within a single manual, including all the documented procedures required by the standard.
- Large, multi-national organizations may need several manuals at the global, national or regional level, and a more complex hierarchy of documentation.
- The quality manual is a document that has to be controlled in accordance with the requirements of clause 4.2.3.
c) Documented procedures:
- ISO 9001:2000 specifically requires the organization to have “documented procedures” for the following six activities:
- 4.2.3 Control of documents
- 4.2.4 Control of records
- 8.2.2 Internal audit
- 8.3 Control of nonconforming product
- 8.5.2 Corrective action
- 8.5.3 Preventive action
- These documented procedures have to be controlled in accordance with the requirements of clause 4.2.3
- Some organizations may find it convenient to combine the procedure for several activities into a single documented procedure (for example, corrective action and preventive action). Others may choose to document a given activity by using more than one documented procedure (for example, Internal audits). Both are acceptable.
- Some organizations (particularly larger organizations, or those with more complex processes) may require additional documented procedures (particularly those relating to product realization processes) in order to implement an effective QMS.
- Other organizations may require additional procedures, but the size and/or culture of the organization could enable these to be effectively implemented without necessarily being documented. However, in order to demonstrate compliance with ISO 9001:2000, the organization has to be able to provide objective evidence (not necessarily documented) that its QMS has been effectively implemented.
d) Documents needed by the organization to ensure the effective planning, operation and control of its processes:
- In order for an organization to demonstrate the effective implementation of its QMS, it may be necessary to develop documents other than documented procedures. However, the only documents specifically mentioned in ISO 9001:2000 are:
- Quality policy (clause 4.2.1.a)
- Quality objectives (clause 4.2.1.a)
- Quality manual (clause 4.2.1.b)
- There are several requirements of ISO 9001:2000 where an organization could add value to its QMS and demonstrate conformity by the preparation of other documents, even though the standard does not specifically require them. Examples may include:
- Process maps, process flow charts and/or process descriptions
- Organization charts
- Specifications
- Work and/or test instructions
- Documents containing internal communications
- Production schedules
- Approved supplier lists
- Test and inspection plans
- Quality plans
- All such documents have to be controlled in accordance with the requirements of clause 4.2.3 and/or 4.2.4, as applicable
e) Records:
- Examples of records specifically required by ISO 9001:2000 are presented in Annex B.
- Organizations are free to develop other records that may be needed to demonstrate conformity of their processes, products and quality management system.
- Requirements for the control of records are different from those for other documents, and all records have to be controlled according to those of clause 4.2.4 of ISO 9001:2000.
Sathis