- Home
- Forums
- Medical Devices, Medical Information Technology, Medical Software and Health Informatics
- Medical Device Related Standards
- ISO 13485:2016 - Medical Device Quality Management Systems
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Design Transfer of medical device
- Thread starter Milas
- Start date
indubioush
Ah ha!
Design transfer activities really depend on your internal processes, whether you are using a contract manufacturer, and other factors. Design transfer doesn't have to be a one-time thing. This list may help though:
Documentation Transferred to Production
Device Specifications
Software Specifications
Subassembly Specifications
Components, Raw Materials Specifications
Packaging Specifications
Labeling Specifications
Environment Specifications
Bill of Materials (BOM)
History Record (HR) Forms/Travelers
Manufacturing Work Instructions
QC Test Instructions and Forms
Tooling/Equipment Specifications
Specifications for Manufacturing Material
Design Transfer Tasks
Process Risk Analysis Performed and Documented
Equipment Installed and Qualified
Suppliers Evaluated and Approved
Product Monitoring Implemented
Process Monitoring Implemented
Supplier Monitoring Implemented
Completion, Approval, Release of DMR
Transfer Conclusion Documented
Documentation Transferred to Production
Device Specifications
Software Specifications
Subassembly Specifications
Components, Raw Materials Specifications
Packaging Specifications
Labeling Specifications
Environment Specifications
Bill of Materials (BOM)
History Record (HR) Forms/Travelers
Manufacturing Work Instructions
QC Test Instructions and Forms
Tooling/Equipment Specifications
Specifications for Manufacturing Material
Design Transfer Tasks
Process Risk Analysis Performed and Documented
Equipment Installed and Qualified
Suppliers Evaluated and Approved
Product Monitoring Implemented
Process Monitoring Implemented
Supplier Monitoring Implemented
Completion, Approval, Release of DMR
Transfer Conclusion Documented
Is there a checklist which shows the deliverables required when a new design is from New Product Development to New Product Introduction ?
Yes: The checklist will be in the design transfer plan for the device.
Just FYI, if that's you, FDA audits will always audit CAPA and Complaints but...the one training record they are keenly interested in is "the person who has final product release authority." Have that person train on all this documentation
Thank you everyone, I have quick follow-up question.
If we take a "typical" medical device development cycle to include the following phases (proof of concept - Engineering Validation - Design Validation - Production validation and commercial build.
During which of the above phases, does the following take place:
Bench test/functional test?
Animal testing?
Clinical Testing?
Regulatory approval?
Any other key milestones?
Thanks
If we take a "typical" medical device development cycle to include the following phases (proof of concept - Engineering Validation - Design Validation - Production validation and commercial build.
During which of the above phases, does the following take place:
Bench test/functional test?
Animal testing?
Clinical Testing?
Regulatory approval?
Any other key milestones?
Thanks
Hi Milas,Thank you everyone, I have quick follow-up question.
If we take a "typical" medical device development cycle to include the following phases (proof of concept - Engineering Validation - Design Validation - Production validation and commercial build.
During which of the above phases, does the following take place:
Bench test/functional test?
Animal testing?
Clinical Testing?
Regulatory approval?
Any other key milestones?
Thanks
i) Bench Test/ Functional Test - It happen during design verification or design validation. We want to ensure that the design output meets the design input by conducting the functional test on the medical device.
ii) Animal Testing/ Clinical Testing - If you are referring to clinical investigation/ clinical trial on human, they would definitely belong to design validation
ii) Regulatory Approval - This process takes place after the process validation, whereby the product has been tested in the pilot run.
You can also refer to the US FDA Design Control for more detail information.
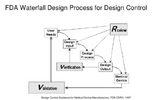
Please can you elaborate on the sottware specfications which need to be transferred, is it just the software version or is there more to it than that ?Design transfer activities really depend on your internal processes, whether you are using a contract manufacturer, and other factors. Design transfer doesn't have to be a one-time thing. This list may help though:
Documentation Transferred to Production
Device Specifications
Software Specifications
Subassembly Specifications
Components, Raw Materials Specifications
Packaging Specifications
Labeling Specifications
Environment Specifications
Bill of Materials (BOM)
History Record (HR) Forms/Travelers
Manufacturing Work Instructions
QC Test Instructions and Forms
Tooling/Equipment Specifications
Specifications for Manufacturing Material
Design Transfer Tasks
Process Risk Analysis Performed and Documented
Equipment Installed and Qualified
Suppliers Evaluated and Approved
Product Monitoring Implemented
Process Monitoring Implemented
Supplier Monitoring Implemented
Completion, Approval, Release of DMR
Transfer Conclusion Documented
indubioush
Ah ha!
It is a requirements for producing the software for use in the medical device. It is everything that defines what it is and how it gets integrated to each system. The specific documentation can vary by company.Please can you elaborate on the software specifications which need to be transferred, is it just the software version or is there more to it than that ?
Two aspects for software in design transfer come to mind:
- How to ensure the correct version of the software is being loaded. What are the controls in place to ensure manufacturing (or whomever) had the right version
- How to ensure the software loaded wasn't corrupted (self-test, checksum)
Similar threads
- Replies
- 1
- Views
- 129
- Replies
- 2
- Views
- 180
- Replies
- 4
- Views
- 415
- Replies
- 3
- Views
- 288
