Hello,
I have a quick question for which I can't seem to find the answer.
In our case we have a reusable class IIa electrical medical device which is packaged into one box (one level of packaging). there are no other level of packaging.
The device has its own UDI-DI ("UDI-DI 1") and the packaging should also have a UDI-DI ("UDI-DI 2") as the UDI-DI is "required for every level of packaging".
My question is : Should the UDI-DI 2 be different than the UDI-DI 1 although there is only one unit inside the packaging ?
I would completely understand if there were many devices in one Packaging unit (UDI-DI 2 would be different to UDI-DI 1) but in our case where we have only 1 device in one packaging unit (so 1:1), I seem to be confused.
I hope that I am clear.
(PS, if you could give also the "source"/requirement for your answer that would be great ! )
Thank you so much.
I have a quick question for which I can't seem to find the answer.
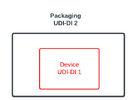
In our case we have a reusable class IIa electrical medical device which is packaged into one box (one level of packaging). there are no other level of packaging.
The device has its own UDI-DI ("UDI-DI 1") and the packaging should also have a UDI-DI ("UDI-DI 2") as the UDI-DI is "required for every level of packaging".
My question is : Should the UDI-DI 2 be different than the UDI-DI 1 although there is only one unit inside the packaging ?
I would completely understand if there were many devices in one Packaging unit (UDI-DI 2 would be different to UDI-DI 1) but in our case where we have only 1 device in one packaging unit (so 1:1), I seem to be confused.
I hope that I am clear.
(PS, if you could give also the "source"/requirement for your answer that would be great ! )
Thank you so much.

