Hi everybody.
I work to a company that will produce software for medical devices. We are at the stage trying to understand and incorporate the I.E.C 62304 to our day to day actions. By reading on the internet is seems that JIRA Software is a good candidate for managing the whole software lifecycle.
So far I have made a specific JIRA workflow for the problem reporting/resolution (see below) procedure.

I was wondering now what kind of workflow will I need to develop for the development part. By default JIRA provides you with the following workflow for the development board.
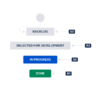
According to your experience, is this sufficient or should be more complex (i.e add code-review, verification steps )? What kind of workflows do you use for that case?
I work to a company that will produce software for medical devices. We are at the stage trying to understand and incorporate the I.E.C 62304 to our day to day actions. By reading on the internet is seems that JIRA Software is a good candidate for managing the whole software lifecycle.
So far I have made a specific JIRA workflow for the problem reporting/resolution (see below) procedure.
I was wondering now what kind of workflow will I need to develop for the development part. By default JIRA provides you with the following workflow for the development board.

According to your experience, is this sufficient or should be more complex (i.e add code-review, verification steps )? What kind of workflows do you use for that case?

