I am trying to design a retro fit QMS and my senior members are getting me in a muddle. Please someone help me
so i have a SOP - procedure, related to the procedure are Forms - which are attachments related to the parent procedure. eg Quality manual has quality policy and objectives as separate forms
SOP-Q01 - Quality manual , this is recorded as a V-01
SOP-Q01-A01 Quality policy and Q01-A02 Quality objectives these are kept as record - YYYYMMDD
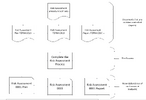
then you have more procedures such as risk assessment SOP-G01 Risk assessment procedure with which you have 3 attachments plan, assessment and report, which all are templates
when completed plan is YYMMDD risk assessment is V1 and report is V1
its is driving me mental. I am trying to keep some control over the template to be used.
A further example is a product stability studies
Plan v1
distribution yyyymmdd
protocol v1
record yyyymmdd
Can you see where the confusion is occurring.
so i have a SOP - procedure, related to the procedure are Forms - which are attachments related to the parent procedure. eg Quality manual has quality policy and objectives as separate forms
SOP-Q01 - Quality manual , this is recorded as a V-01
SOP-Q01-A01 Quality policy and Q01-A02 Quality objectives these are kept as record - YYYYMMDD
then you have more procedures such as risk assessment SOP-G01 Risk assessment procedure with which you have 3 attachments plan, assessment and report, which all are templates
when completed plan is YYMMDD risk assessment is V1 and report is V1
its is driving me mental. I am trying to keep some control over the template to be used.
A further example is a product stability studies
Plan v1
distribution yyyymmdd
protocol v1
record yyyymmdd
Can you see where the confusion is occurring.