According to cl.3.8 of IEC 60601-1, the definition of APPLIED PART is “part of ME EQUIPMENT that in NORMAL USE necessarily comes into physical contact with the PATIENT for ME EQUIPMENT or an ME SYSTEM to perform its function”
An internally powered auto-injector, which allowed patients to operate by themselves, for example in the below photo, has an injection tip (orange color part) that will contact injected skin tissue. And that tip is considered as APPLIED PART undoubtedly. If we stick to the literal content of cl.3.8, the handheld part (white color part) of the auto-injector, which came into physical contact with the PATIENT(=OPERATOR), will also fall into the definition of APPLIED PART.
Then, the manufacturer want to apply IEC 60601-1-11 cl.8.5.3 second paragraph "However, parts which the PATIENT intentionally handles as the intended OPERATOR according to 7.9.2.1 of IEC 60601-1:2005 and IEC 60601-1:2005/AMD1:2012 + IEC 60601-1:2005/AMD2:2020 (e.g. touch keys, ENCLOSURE) while the ME EQUIPMENT is not being used for its intended medical function may be insulated with two MOOP from SUPPLY MAINS." And the manufacturer want to treat the handheld part (white color part) as ACCESSIBLE PART, instead of APPLIED PART. And treat it as MOOP protected ACCESSILBE PART.
My question is the following phrase "while the ME EQUIPMENT is not being used for its intended medical function." Is the clause support to treat handheld part (white color part) as MOOP protected ACCESSILBE PART, while the duration the patient is injecting him/herself?
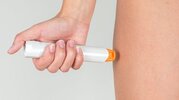
An internally powered auto-injector, which allowed patients to operate by themselves, for example in the below photo, has an injection tip (orange color part) that will contact injected skin tissue. And that tip is considered as APPLIED PART undoubtedly. If we stick to the literal content of cl.3.8, the handheld part (white color part) of the auto-injector, which came into physical contact with the PATIENT(=OPERATOR), will also fall into the definition of APPLIED PART.
Then, the manufacturer want to apply IEC 60601-1-11 cl.8.5.3 second paragraph "However, parts which the PATIENT intentionally handles as the intended OPERATOR according to 7.9.2.1 of IEC 60601-1:2005 and IEC 60601-1:2005/AMD1:2012 + IEC 60601-1:2005/AMD2:2020 (e.g. touch keys, ENCLOSURE) while the ME EQUIPMENT is not being used for its intended medical function may be insulated with two MOOP from SUPPLY MAINS." And the manufacturer want to treat the handheld part (white color part) as ACCESSIBLE PART, instead of APPLIED PART. And treat it as MOOP protected ACCESSILBE PART.
My question is the following phrase "while the ME EQUIPMENT is not being used for its intended medical function." Is the clause support to treat handheld part (white color part) as MOOP protected ACCESSILBE PART, while the duration the patient is injecting him/herself?